All through the brewing process, having your mash liquor, wort and proto-beer within the desired pH window is critical to both quality and yield. When we are mashing or fermenting we are relying on enzymes which work best within a tight pH window. During boiling it is chemistry rather than biochemistry which is at play, but still pH is critical to the final beer quality.
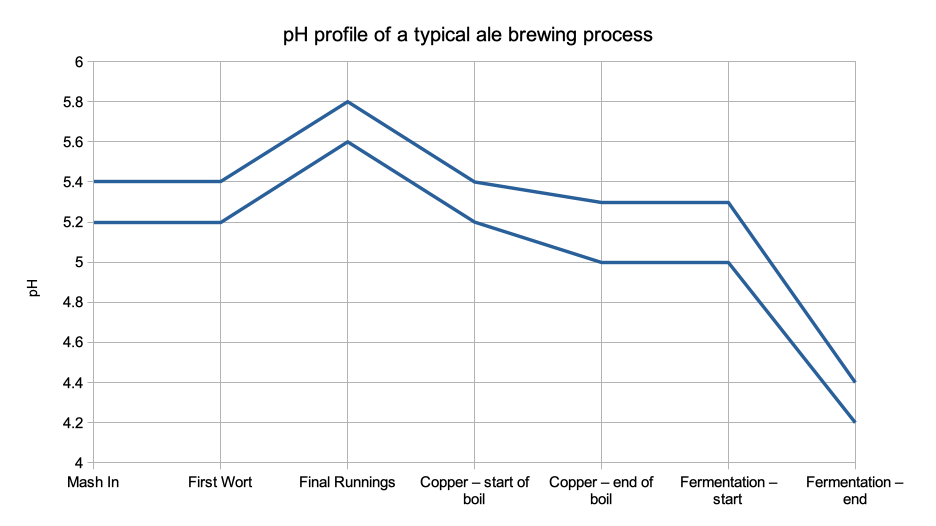
The chart below shows the ideal pH window as it tracks through the whole brewing process.
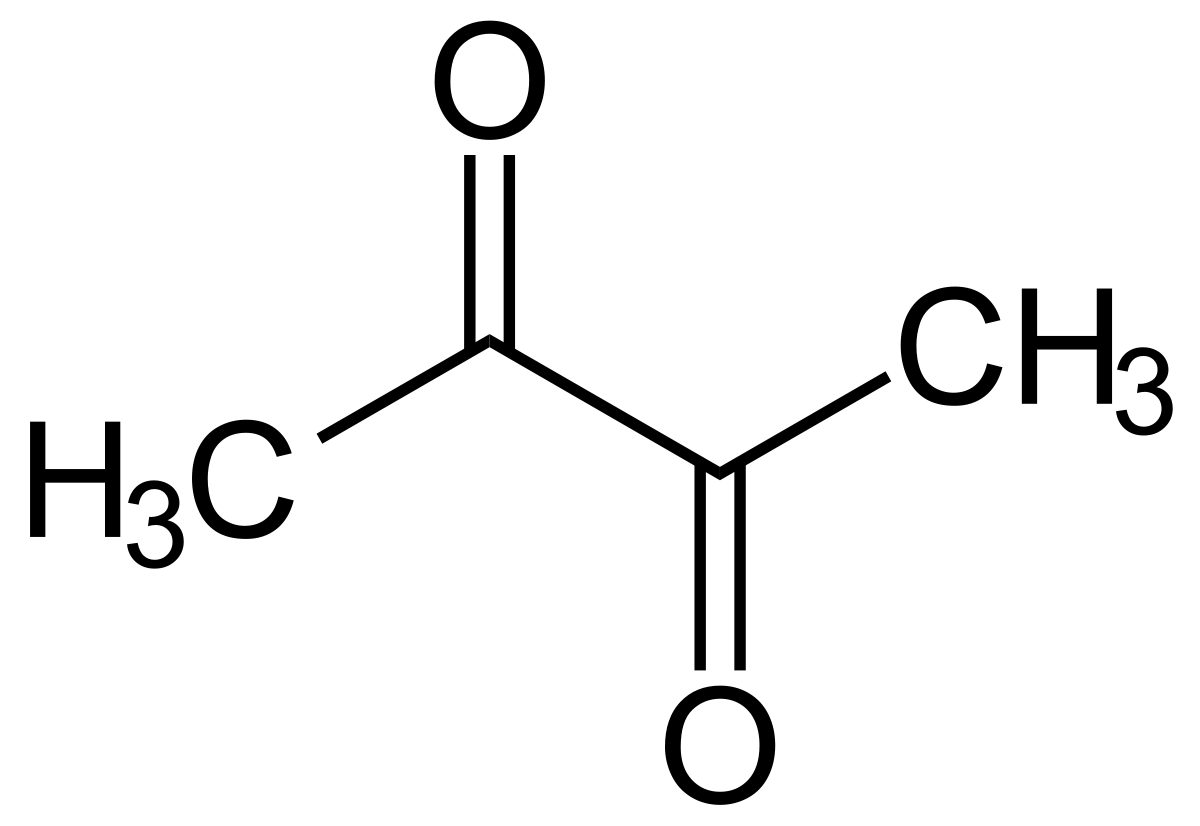
Mashing
During the mash we want the α and β amylases to work as effectively as possible and that means that the mash should be between pH 5.2 and 5.4. This is controlled primarily by your water chemistry which I covered in an earlier post. If your pH is too low your starch conversion efficiency will drop, if it’s too high you’ll start to extract tannins and silicates which you want to leave behind in the spent grain. If you are running a new recipe it’s wise to check this pH on the first couple of runs to make sure it within range. This is doubly important with darker beers, where the roasted malts can have a big impact on the pH. There are ways to estimate this, but they are only estimates and it’s best to check what you’ve achieved and adjust your calcium or bicarb addition levels on porters and stouts. After that it’s good to check your run off pH once a week / fortnight to check nothing has drifted.
That astringency that you taste in some stouts, it shouldn’t be there! That is someone not properly attending to their mash pH.
Mashing is the stage where pH is most critical, if you get inside the correct window here then it should track within the desired window for the rest of the process for beers above 3.0% ABV.
Sparging
Here you should be using either pH or SG to monitor your last runnings so as not to go too far. That last portion of weak worts doesn’t contain a whole lot of sugar but will contain undesirable levels of polyphenols or lipids if you let the pH go above 5.8 or SG below 1.006. Remember you are sparging with water which will be pH 7 or slightly above, so the pH of the run off will start to rise from the middle of the sparge onwards
Boiling
There’s a lot that goes on in the boil. The pH will impact the colour development, hop α-acid isomerisation and protein drop out (thus final haze stability). Different pH’s are ideal for each, so the key here is consistency. The pH drops during the boil due to the acid end products of the Maillard reaction and more Ca phosphate precipitation. If necessary you can add a little phosphoric or lactic acid during the boil if the pH is outside of your normal range. However if you have got your mash pH correct and not over-sparged you shouldn’t have an issue. If you are making a low alcohol beer you are likely to need to add a little acid to drop the pH into range.
Fermentation
Again we are trying to keep the enzymes happy and so long as we’ve done everything right up to this point all should be well. Yeast produces acids as a by-product of cell growth and also as one of it’s sugar transport mechanisms. One key thing to remember is to drop the yeast, or transfer the beer off the yeast in a timely fashion or you’ll see your pH rise, reducing shelf life as well as adding undesired ‘beefy’ flavours to your beer. If you are making a low alcohol beer again extra care should be taken and the pH adjusted if necessary.
pH measurement
After spending 20 years in the chemical industry I know more than a little about temperamental pH meters. If you are going to buy a pH meter don’t skimp on price or pick the cheapest one you can find on Amazon. These will never be as accurate or repeatable as you’ll need for brewing. Something from Myron or a bench based model from Jenway would be good options. If you cannot spend £250 then your better option would be to get some narrow range pH test sticks (try these or these). These may not be as precise but if you want something inexpensive yet accurate they are your very best option. You can know for sure if you are sitting inside your desired range or when to stop your sparge. They are quick too.